Working Principle
Hydrochloric acid and water form a maximum boiling point azeotrope at 11O°C corresponding to a concentration of 20.24%; (w/w) HCl. By adding concentrated CaCl solution to commercial hydrochloric acid the azeotrope 2 point is eliminated and the entire' HCl becomes available for liberation by distillation. Anhydrous HCl gas generation through Calcium Chloride Route is the most environmental friendly technique.
Process Description
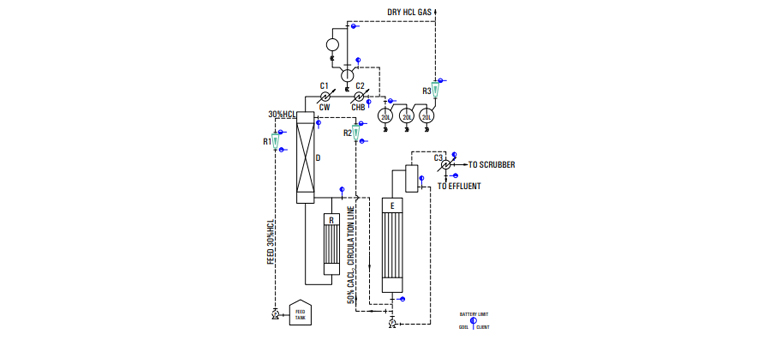
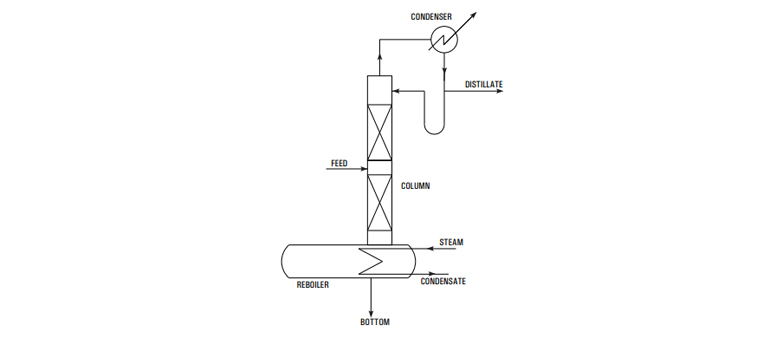
The above principle- is achieved in practice by feeding metered quantities of commercial HCl and 50% CaCl -solution to a stripping column with a steam 2 heated re-boiler at bottom. The effluent from bottom of the column is a dilute acidic calcium chloride solution which is concentrated to 50% in a evaporator and re-used. The vapor leaving is condensed stage wise with cooling water and chilled brine as coolant. The relatively dry gas passes through a mist eliminator and then through a rotameter to the point of consumption.
Raw material utility requirements
The indicative requirements for 20 Kg/hr HCl gas generator are given below
- 30-32 % HCl, (Kg/hr) : 66
- Cooling water at 30 °C (M /hr) : 4
- Chilled brine at -10 °C (M /hr) : 3
- Steam at 6 Kg/cm (g) : 150
| LEGEND |
|---|
| R - REBOILER |
| D - COLUMN |
| E - EVAPORATOR |
| C1 - PRIMARY CONDENSER |
| C2 - SECONDARYCONDENSER |
| R1 - FEED HCL ROTAMETER |
| R2 - FEED CACL2 ROTAMETER |
| R3 - DRY HCL GAS ROTAMETER |
| CW - COOLING WATER |
| CHB - CHILLED BRINE |
| C3 - CONDENSER |
| P - PUMP |